How to create a new version of a CRF
Login to OpenClinica as Study Director and click on "Tasks". Choose from the list that appears "CRF's", under header "Administration" (fig. 1).
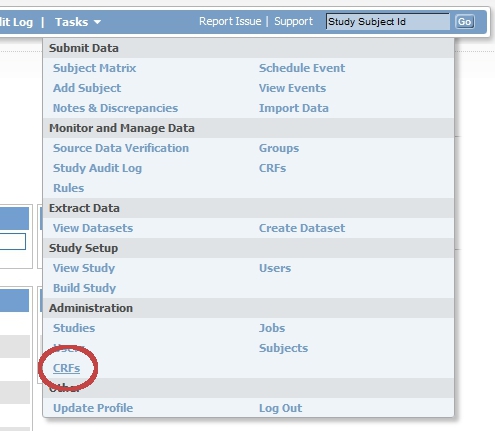
fig. 1: Task menu: CRF's
In the screen that now appears, "Administer Case Report Forms (CRF's)" find the CRF you want to revise and click on the white arrow in the "Download" column (fig. 2). Clicking this link starts a down-load of the Excel-sheet.
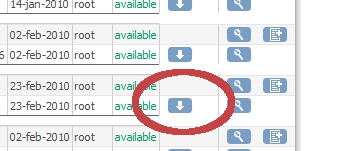
fig. 2: Download CRF
Save this Excel-sheet on a your computer. Open it in Excel and start with the first Tab "CRF" and fill in a new version number in cell B2
Now do your other editing and save the file.
Switch back to OpenClinica "Administer Case Report Forms (CRF's)" and in the right column, titled "Actions" click on the icon labeled "Create New Version" (fig. 3)
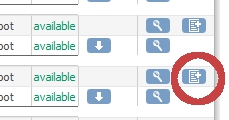
fig. 3: Create a new version-a
You get a screen very similar to that of creating a new CRF, but this one is titled "Create a CRF Version for X" (fig. 4). Click the button labeled Browse and browse to the location on your PC where you saved the XL-sheet. Click on "Preview CRF Version" to have the CRF validated.
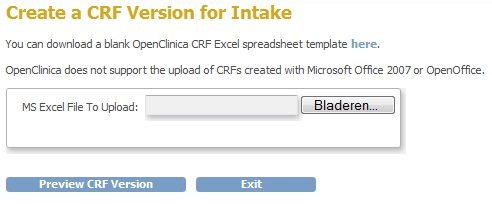
fig. 4: Create a new version-b
Consequences of having a new version
If no errors where found, the CRF will be included in your list of available CRF's.
If the previous version of this particular CRF was never used you can overwrite it:
you keep the version in cell B2 the same. On validating you will see a message asking
you if it's OK to overwrite and you can decide what to do with that.
In all other circumstances you now have two or more versions of a CRF. This means that you have to define
for each Event that uses this CRF, which version is the default version!
This may seem trivial, but that's not so when you have more studies using the same CRF. If you decide you want to have a new version for one study, this applies to all: maybe a bit more than you want. Therefore it is a good thing to decide, when you design a new study and decide you can use some of the existing CRF's whether you want to use these CRF's, or make a copy of them and you the copies in your new study.
Another not so trivial consequence of having a new version of your CRF is that you must rewrite all rules associated with the CRF, because the OID of it has changed! This can be quite a task, as you can not download a "version" of a rule, edit it and upload it again. You will have to keep your own administration of the rules.